Avient Announces Expansion of NEUSoft™ TPU Manufacturing to China at Medtec China 2024, Enhancing Service for APAC Customers
September 25, 2024
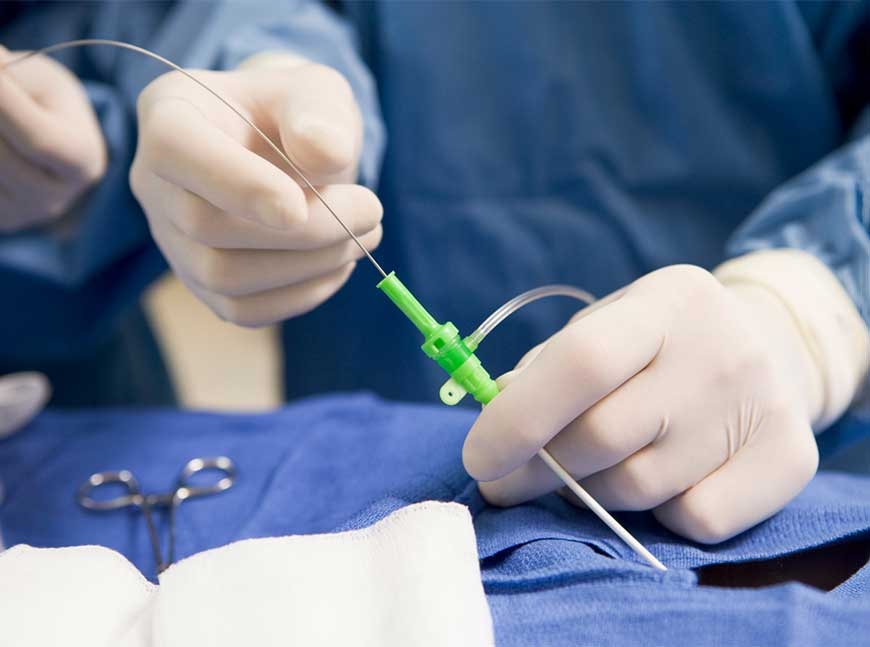
Avient Announces Healthcare TPU Manufacturing Expansion and
Showcases Specialty and Sustainable Materials for Healthcare at Medtec China 2024
To download a high-resolution image, please click here: FOR MEDIA USE ONLY
SHANGHAI– September 25, 2024 – Today, Avient Corporation, a premier provider of specialty and sustainable materials solutions and services, announced it is expanding the manufacturing of its NEU™ Custom Capabilities and NEUSoft™ Thermoplastic Polyurethane (TPU) solution for catheter applications to its plant in Suzhou, China. This announcement also coincides with the start of Avient’s exhibit at Medtec China 2024 today, one of the country’s top medical technology expositions.
"By bringing NEU Custom Capabilities and NEUSoft TPU production to China, we're reaffirming our commitment to serving the growing medical materials demands from our healthcare and medical device customers in China and the Asia-Pacific (APAC) region," said Matt Mitchell, Director, Global Marketing of Specialty Engineered Materials at Avient, "This development not only helps us to improve our response time but also allows us to work more closely with our customers, providing customized solutions that meet their specific needs."
Avient’s NEU business has provided catheter material solutions to the healthcare market for 35 years. The NEUSoft TPUs are a range of ultra-low durometer formulations for short-term in-vivo catheter applications, including cardiovascular, intravenous, and other specialty segments. They provide excellent elasticity, resistance to abrasion and tear, strong ultraviolet (UV) stability, and barrier properties against moisture and oxygen. They are also certified to meet biocompatibility requirements for ISO 10993 and USP Class VI standards. The materials can also be customized with radiopacifiers, colors, and other additives to meet specific application requirements.
In addition, the material can be overmolded on various substrates such as TPU and Nylon to allow for the creation of complex medical devices. Meanwhile, its compatibility with high-speed processing methods such as extrusion and injection molding provides additional flexibility that enables manufacturers to develop multi-material medical devices more efficiently.
Avient's Suzhou facility operates under ISO 13485 quality management system certification, complying with the leading standards in medical device manufacturing. The plant features climate-controlled environments, improved operational flow to maintain product integrity and consistency, and a specialized production room.
Avient will exhibit its NEUSoft solutions and other specialty and sustainable materials for healthcare at Medtec China 2024 in Hall H2, Booth No. 2D411, at the Shanghai World Expo Exhibition and Convention Center from September 25 to 27, 2024.
For more information about NEUSoft TPU and Avient's healthcare solutions, visit www.avient.com or contact NEU@Avient.com.
About Avient
Avient Corporation provides specialized and sustainable materials solutions that transform customer challenges into opportunities, bringing new products to life for a better world. Examples include:
- Dyneema®, the world's strongest fiber™, enables unmatched levels of performance and protection for end-use applications, including ballistic personal protection, marine and sustainable infrastructure and outdoor sports
- Unique technologies that improve the recyclability of products and enable recycled content to be incorporated, thus advancing a more circular economy
- Light-weighting solutions that replace heavier traditional materials like metal, glass and wood, which can improve fuel efficiency in all modes of transportation and reduce carbon footprint
- Sustainable infrastructure solutions that increase energy efficiency, renewable energy, natural resource conservation and fiber optic / 5G network accessibility
Avient is certified ACC Responsible Care®, a founding member of the Alliance to End Plastic Waste and certified Great Place to Work®. For more information, visit www.avient.com.
To access Avient’s news library online, please visit www.avient.com/news.
# # #
Media Contact
Jennifer Huang
Senior Manager, Marketing Communications Asia
+86 21 6028 4888
jennifer.huang@avient.com
Press Resources
Investor News
Avient Announces Quarterly Dividend... More
Avient Announces Fourth-Quarter and Full-Year 2025 Results; Initiates Full-Year 2026 Financial Guidance... More
Avient To Hold Fourth Quarter 2025 Conference Call... More